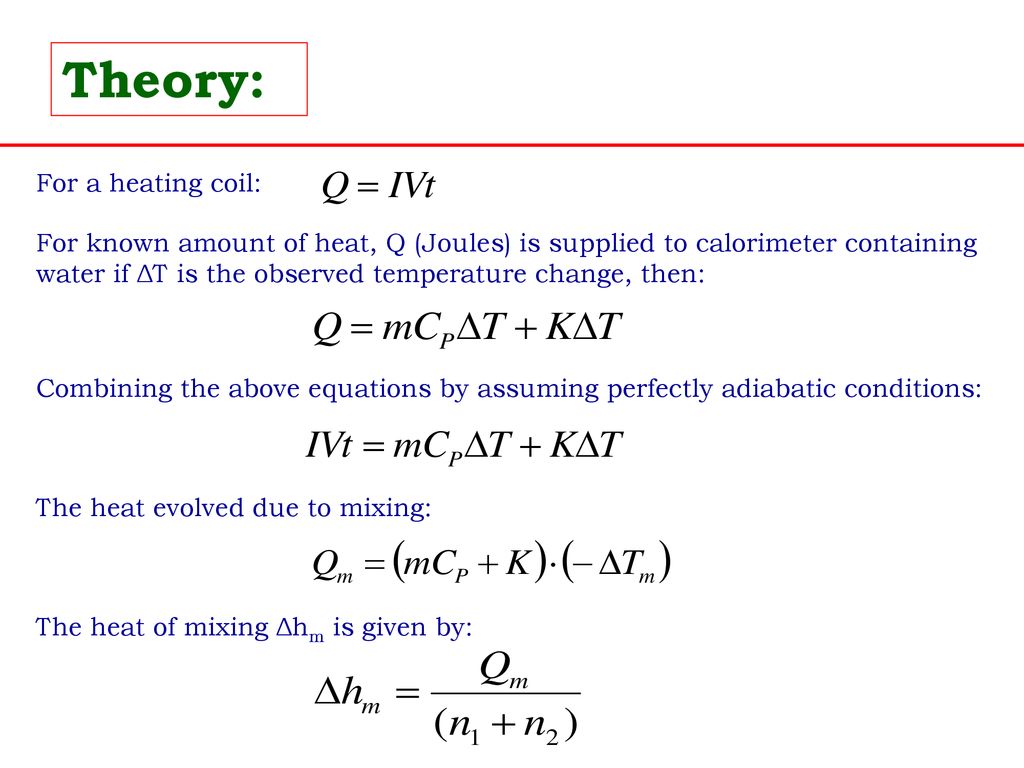
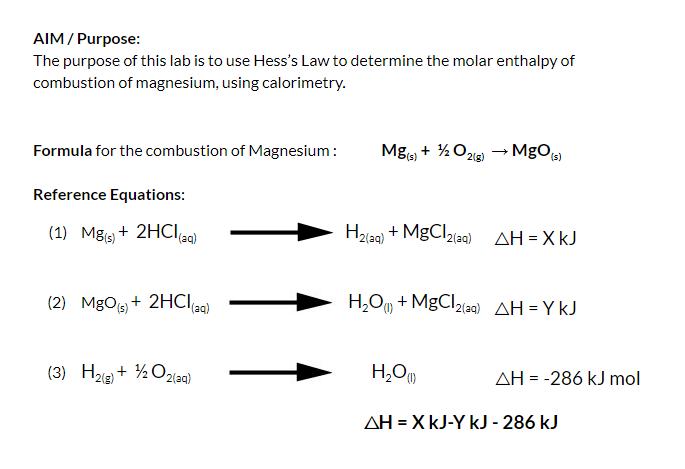
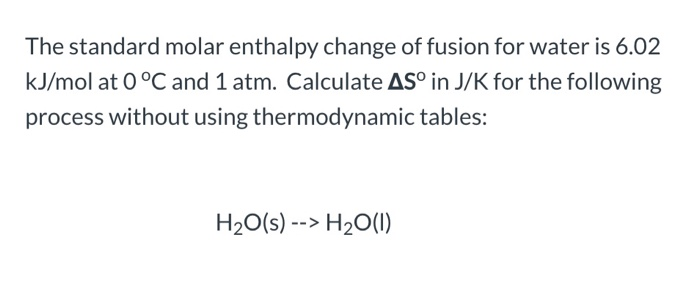
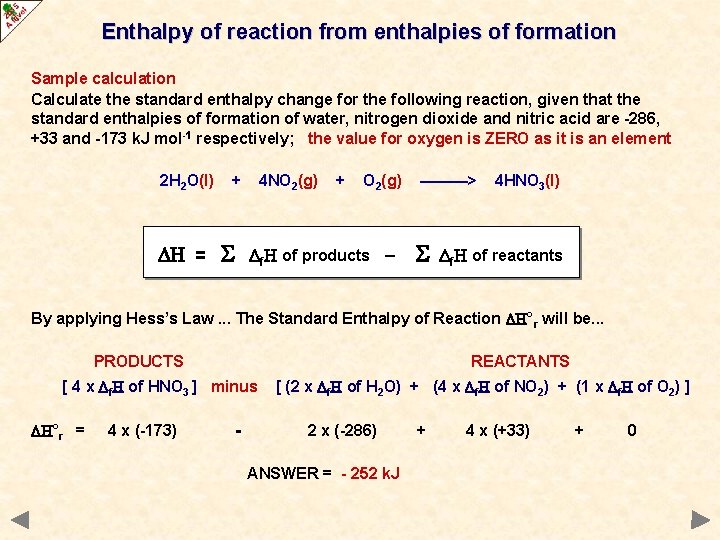
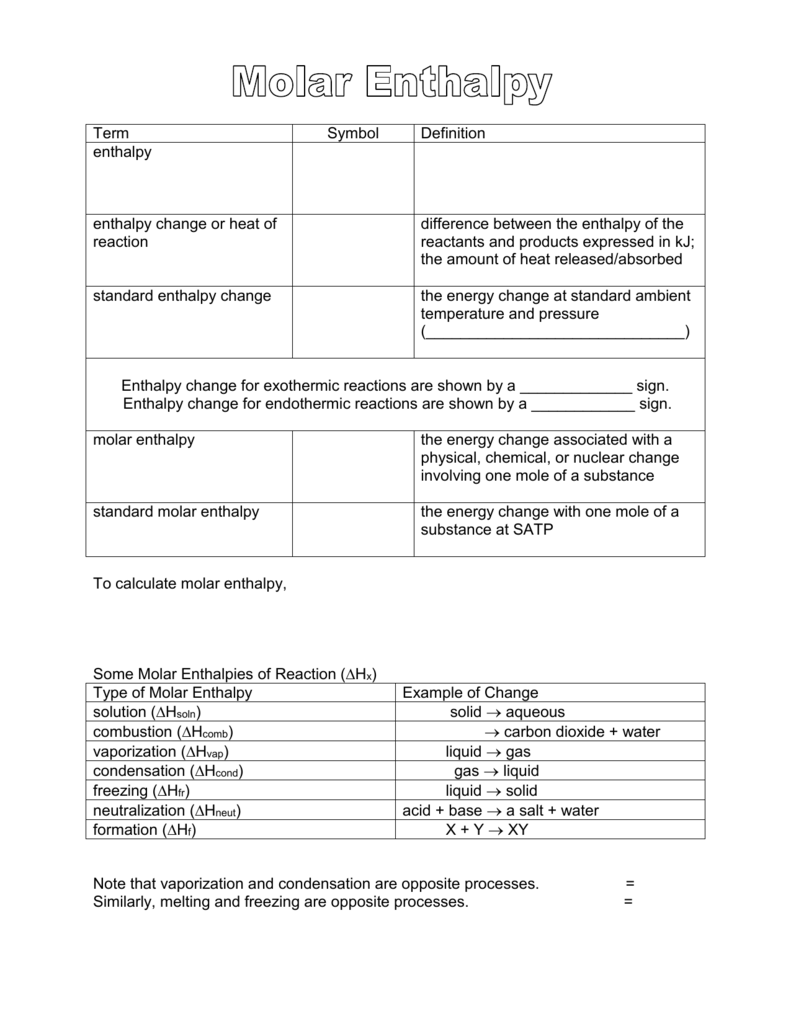
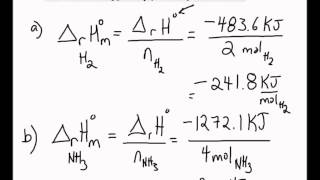Experiment: To measure the molar enthalpy change for the dissolution ( enthalpy of solution) for vari - Tutorke

Calculate the change in molar entropy and change in Gibbs' free energy when 1 mol of liquid butanol vaporizes at 25ºC to a gas that is at 0.001 atm? | Socratic
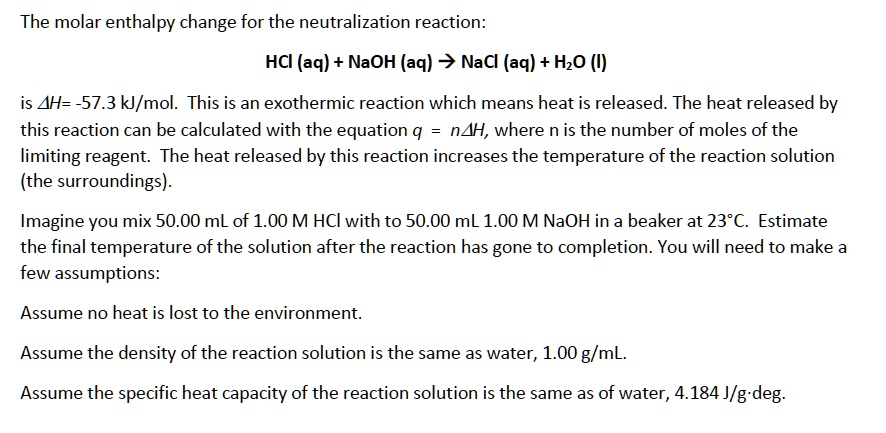
SOLVED:The molar enthalpy change for the neutralization reaction: HCI (aq) + NaOH (aq) +> NaCl (aq) + HzO (I) is AH=-57.3 k/mol. This is an exothermic reaction which means heat is released.
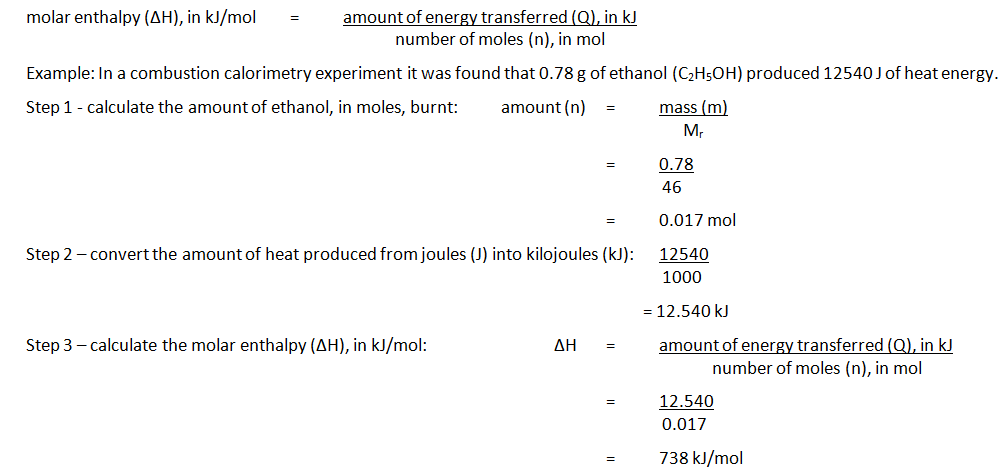
3:04 calculate the molar enthalpy change (ΔH) from the heat energy change, Q - TutorMyself Chemistry

The molar enthalpy of neutralization was experimentary shown to be 51.5kJ per mole of 0.5M hydrochloric acid and 0.5M sodium hydroxide. If the volume of...
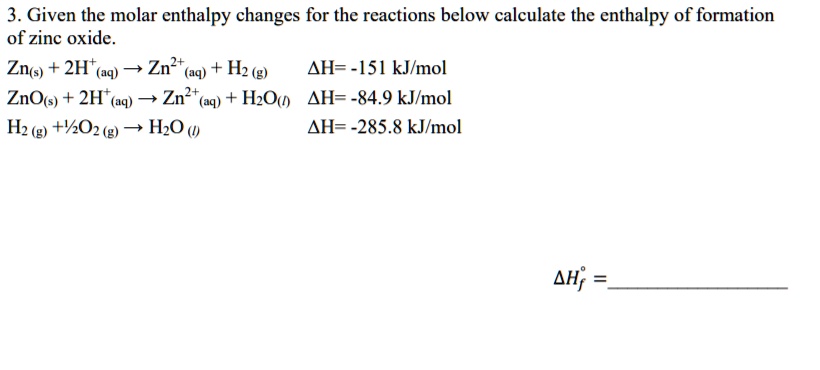
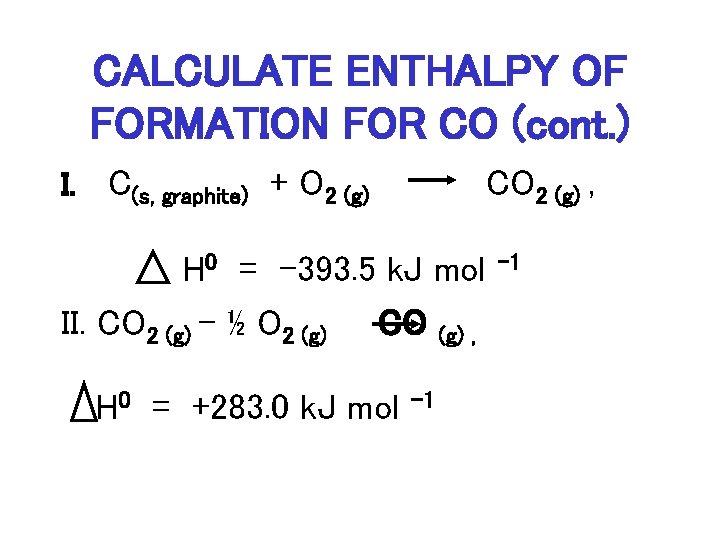
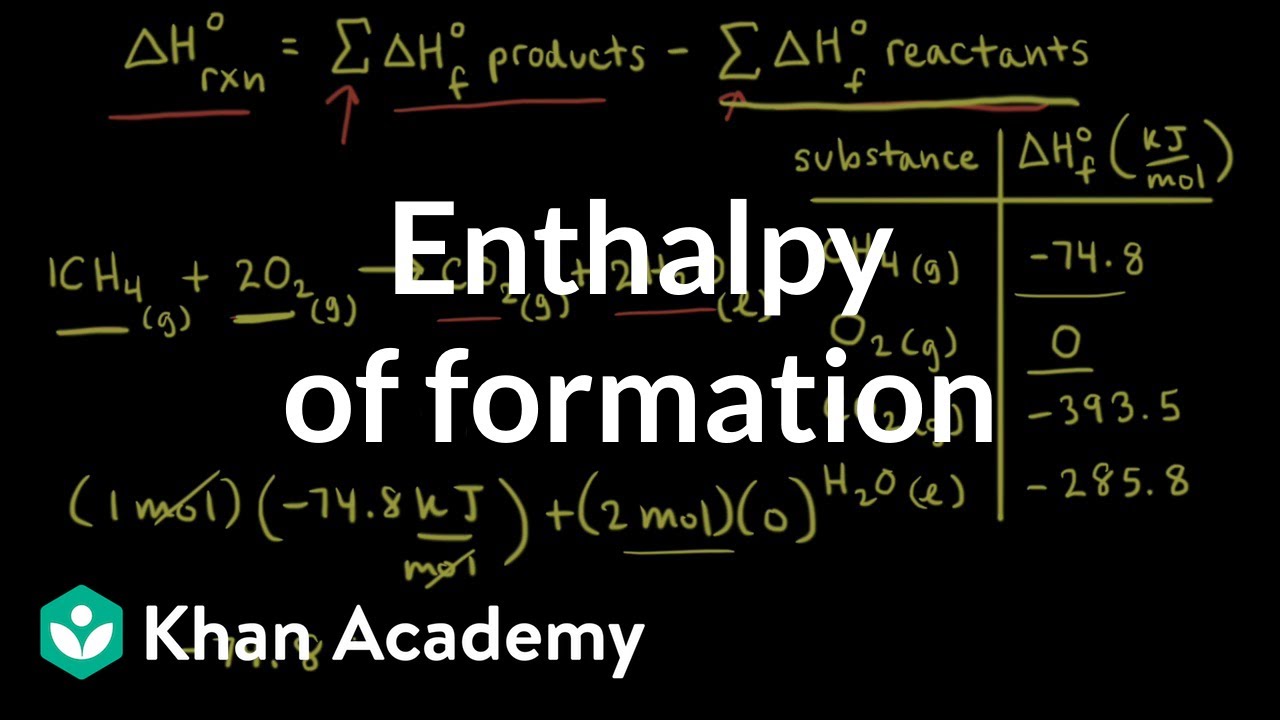
SOLVED:3. Given the molar enthalpy changes for the reactions below calculate the enthalpy of formation of zinc oxide_ Zno) + 2Ht(aq) ~> Zn?t(aq) + Hz (g) AH=-151 kJlmol ZnOts) + 2Ht(aq) ~>